
The Highest Standards from Tissue Recovery through Processing
At Pinnacle, safety and quality are our priority. We maintain the highest quality standards from recovery through processing to ensure safety for our employees and patients. Pinnacle is registered with the Food and Drug Administration (FDA), accredited by the American Association of Tissue Banks (AATB) and maintains all applicable state licenses.
The Highest Standards from Tissue Recovery through Processing
At Pinnacle, safety and quality are our priority. We maintain the highest quality standards from recovery through processing to ensure safety for our employees and patients. Pinnacle is registered with the Food and Drug Administration (FDA), accredited by the American Association of Tissue Banks (AATB) and maintains all applicable state licenses.

Tissue Safety & Quality
1. Tissue Recovery
Recovery or collection of all donated tissues is performed by our recovery partners across the United States using surgical procedures to minimize contamination and bioburden.

2. Donor Screening
Each donor is subjected to a thorough eligibility evaluation (i.e. a donor risk assessment interview with the family, medical records review, infectious disease screening, autopsy report if performed and physical assessment)

3. Serological Testing
As part of the donor screening process, each donor undergoes an extensive panel of serological and microbiological tests. Laboratory testing is performed in a CLIA certified laboratory and includes, but is not limited to, the following:
- HBsAg: Hepatitis B Surface Antigen
- HBcAb: Hepatitis B Core Antibody
- HCVAB: Hepatitis C Antibody
- HCV NAT: Hepatitis C Virus
- HBV NAT: Hepatitis B Virus
- HIV NAT: Human Immunodeficiency Virus
- HIV 1/2/O Ab: Human Immunodeficiency Virus 1/2 & 0 Antibody
- RPR/STS or Equivalent: Syphilis
- WNV NAT: West Nile Virus (Birth Tissue)
- HTLV I/II Ab: Human T-Cell Lymphotropic Virus Antibodies (Birth Tissue)

4. Donor Eligibility
Based on all screening and testing results, donor tissue is determined to be suitable for transplant by our Medical Director and Quality Assurance Team.

5. Tissue Processing
Tissue is subjected to a proprietary series of disinfection soaks and mechanical agitation to decontaminate the tissue, remove blood and lipids, and reduce bioburden. Tissue is cleansed again using a sterile rinse to remove any residual processing reagents, bloods or lipids.
6. Tissue Sterilization
Pinnacle's tissue sterilization process allows bone & soft tissue to maintain biomechanical integrity while achieving a sterility assurance level (SAL) of 10-6.
Tissue Safety & Quality

1. Tissue Recovery
Recovery or collection of all donated tissues is performed by our recovery partners across the United States using surgical procedures to minimize contamination and bioburden.

4. Donor Eligibility
Based on all screening and testing results, donor tissue is determined to be suitable for transplant by our Medical Director and Quality Assurance Team.

2. Donor Screening
Each donor is subjected to a thorough eligibility evaluation (i.e. a donor risk assessment interview with the family, medical records review, infectious disease screening, autopsy report if performed and physical assessment)

5. Tissue Processing
Tissue is subjected to a proprietary series of disinfection soaks and mechanical agitation to decontaminate the tissue, remove blood and lipids, and reduce bioburden. Tissue is cleansed again using a sterile rinse to remove any residual processing reagents, bloods or lipids.

3. Serological Testing
As part of the donor screening process, each donor undergoes an extensive panel of serological and microbiological tests. Laboratory testing is performed in a CLIA certified laboratory and includes, but is not limited to, the following:
- HBsAg: Hepatitis B Surface Antigen
- HBcAb: Hepatitis B Core Antibody
- HCVAB: Hepatitis C Antibody
- HCV NAT: Hepatitis C Virus
- HBV NAT: Hepatitis B Virus
- HIV NAT: Human Immunodeficiency Virus
- HIV 1/2/O Ab: Human Immunodeficiency Virus 1/2 & 0 Antibody
- RPR/STS or Equivalent: Syphilis
- WNV NAT: West Nile Virus (Birth Tissue)
- HTLV I/II Ab: Human T-Cell Lymphotropic Virus Antibodies (Birth Tissue)
6. Tissue Sterilization
Pinnacle's tissue sterilization process allows bone & soft tissue to maintain biomechanical integrity while achieving a sterility assurance level (SAL) of 10-6.
OUR PRODUCTS
Allograft Portfolio
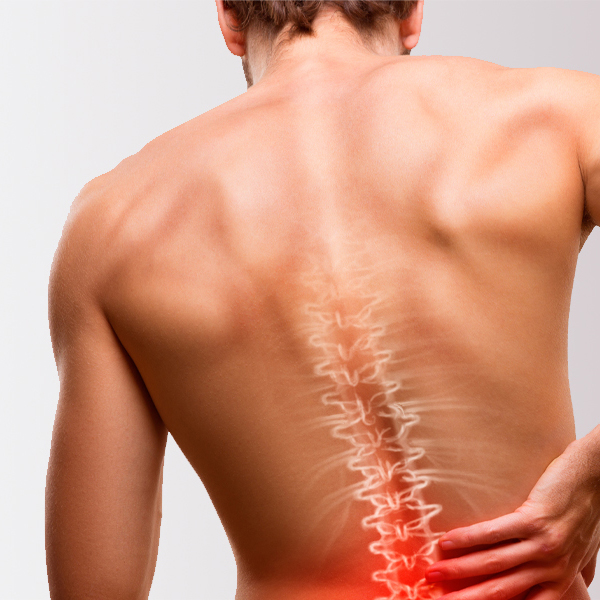
Spine Allografts
Precision-machined grafts, demineralized bone matrix/fibers (DBM/DBF) and allograft chips
Sports Medicine Allografts
Soft tissue allografts, with and without bone
Wound Care Allografts
Biological overlay for wound healing
Orthopedics Allografts
Versatile solutions for a wide array of orthopedic applications
Contact Us
Pinnacle is committed to honoring the gift of donation and improving patient’s quality of life through the processing and distribution of high-quality allograft implants.
Customer Service
1125 W. Pinnacle Peak Road, Building #2
Phoenix, AZ 85027
Phone: 623-277-5400
Fax: 623-277-5401
info@pinnacletransplant.com
To request more information or contact a representative from Pinnacle’s Team, please complete the form below

